
Nanjing RegeneCore Biotech Co.,Ltd
Nanjing RegeneCore Biotech Co.,Ltd is established in the Biomedical Valley of National Nanjing Jiangbei New Area, with a research and office base of nearly 4000 square meters. It is one of the first research and development institutions in the field of nanobodies in China. The company's international scientific consulting expert team has rich experience in the research and development of cell therapy products and clinical applications, providing strong guarantees for the precise positioning and success rate of research and development products. The company has mature and leading nanobody screening technology, and conducts in-depth development in multiple application fields based on this technology.

NEWS
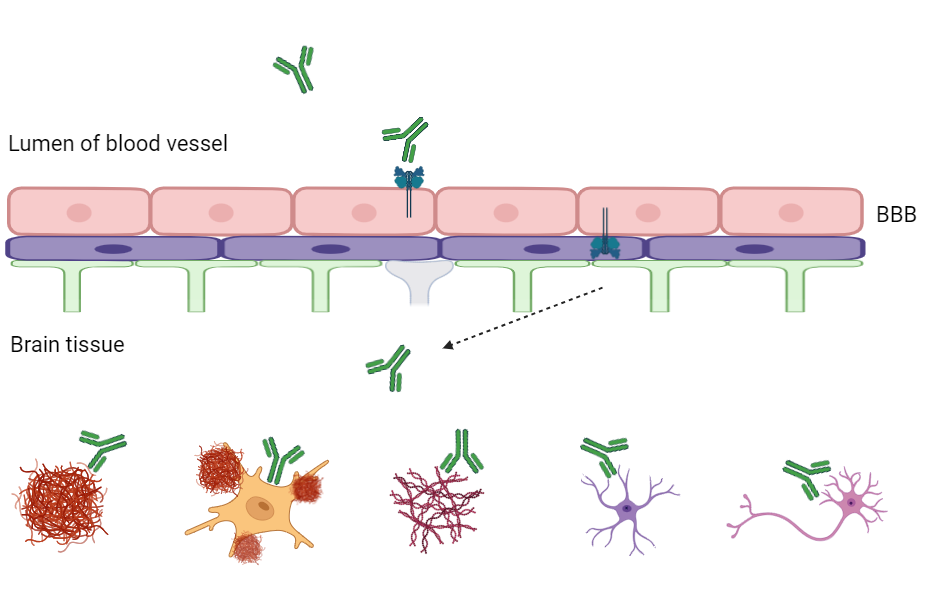
Nanjing RegeneCore Announces New Breakthrough in Brain-Penetrating Drug Delivery Technology
Nanjing Rongjiekang RC1416 dual antibody has obtained 2 clinical trial licenses again
YOUNGY GROUP: Racing to New Frontiers and Scaling New Heights in Life Sciences
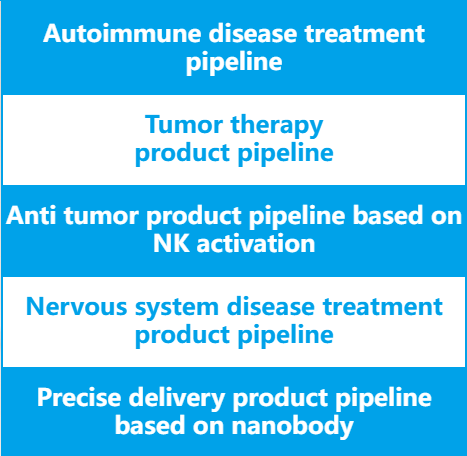
Platforms
High throughput nanobody screening technology platform, nanobody humanization technology platform, antibody immunogenicity detection technology platform, biomacromolecule analysis technology platform, antibody drug CMC process development technology platform
TEL:
Address: Room 07 Building 16 Treehouse, No. 73, Tanmi Road, Jiangbei New District, Nanjing
Enterprise email:rjk@regenecore.com

WeChat cooperative consultation
You are the th visitor




 025-58608860
025-58608860 

 Contact
Contact 